Beijing Zhongkebaike Biotechnology Co., Ltd., as a global leader in the field of pet interferon, followed the pace of "Zhongguancun Going Global" and came to Beijing-Tianjin Zhongguancun Science and Technology City.
Beijing Zhongkebaik Biotechnology Co., Ltd. was established in 2007. In the early days of its establishment, it mainly focused on the sales of veterinary drugs. In order to improve the company's competitive advantage, in August 2010, the company established a new branch in Zhongguancun Life Science Park. Zhongke Baik Pharmaceutical Research Institute has increased investment in research and development and successfully transformed the company into a high-tech company with technology research and development as its core, specializing in the research and development and promotion of pet drugs, aiming to provide clinical veterinarians with the most professional and high-quality products. Technical Services.
The size of Zhongkebaike’s market technical service team is in the leading position in the industry. Currently, the technical team includes 2 doctors, 11 masters, and 15 undergraduates. Their majors include molecular biology, cell biology, protein engineering, and pharmacy. and many other fields. The company has been rated as a national high-tech enterprise for ten consecutive years. It currently has 3 invention patents and has many other patents in the process of application. In November this year, it obtained a new drug certificate for recombinant canine alpha interferon (lyophilized type) - (2019) New Veterinary Drug Certificate No. 64. "This is the result of the company's seven years of hard work, and it is also a milestone achievement for the company." Chen Huangshi said, "At the same time, Zhongkebaik also has 16 new drug projects that are being registered and developed, which will be launched in the next few years. Obtained the new drug certificate within the year”

When talking about why he chose Zhongguancun in the early stage of starting a business, Deputy General Manager Chen Huangshi said: "Zhongguancun, Beijing, has intensive scientific and technological conditions, scientific and technological talents, scientific and technological achievements, and scientific and technological enterprises. It is a professional incubation base for small and medium-sized biomedical enterprises and the first choice for biomedical innovation and entrepreneurship in the country. Zhongkebaike is a technology-based small and medium-sized enterprise engaged in the field of biomedicine. The soil of Zhongguancun is particularly suitable for our development. "< /p>
The Zhongguancun Life Science Park (hereinafter referred to as the "Life Park"), where Zhongkebaike was initially settled, was born in 1999 when the State Council issued the "Reply on Issues Concerning the Construction of Zhongguancun Science and Technology Park" and the "Beijing Ten-Year Plan" in 2000. Against the historical background of the approval of the Science and Technology Development Plan for the Five-Year Period. In the past 20 years, Life Park has adhered to the mission of building a high-quality "innovation ecosystem" and providing high-quality software and hardware services to entrepreneurs. It has successfully attracted more than 200 high-tech biopharmaceutical industries to settle in, cultivated ten listed companies, and achieved an incubation success rate of Up to 80%.
“There are very few antiviral biological products for pets in China, especially broad-spectrum antiviral drugs like interferon.” When introducing the company’s star products, Chen Huangshi A light appeared in his eyes. Interferon is a group of active glycoproteins with multiple biological functions. It is mainly stimulated by animal cells under the action of inducers such as viruses. It is a type of cytokine with anti-viral, anti-proliferative and immunomodulatory activities. In 1986, the world's first recombinant human interferon (IFN-α2b) was launched by the American Schering Plow Company and approved by the FDA for the treatment of chronic hepatitis B. In 1992, domestically produced recombinant human interferon α1b was launched. But so far, there are no official interferon products for dogs in the world. The recombinant canine alpha interferon for injection (lyophilized type) developed by Zhongkebaike is a human-to-veterinary drug with broad-spectrum antiviral effects and huge market value.
It is understood that the current purity standard for veterinary drugs is 95%, while the purity of Zhongkebaike Interferon is as high as 98%, with very low impurity protein content and almost no allergies. It can be safely used during pregnancy and lactation. animals or young and old animals. What's more, because it adopts the most advanced non-terminal sterilization low-temperature freeze-drying process and strict to almost harsh quality control, the product far exceeds the national standard and represents the highest standard of veterinary biological products.
According to Chen Huangshi, the pharmaceutical industry has its own particularities, with relatively high registration thresholds and very strict technical requirements. It takes 5-6 years for a drug to go from project establishment to obtaining a certificate, during which it has to go through a number of key approval links. Take Zhongke Baike Dog Interferon as an example. From the beginning of the project, it must obtain the "Genetically Modified Organism Safety Certificate" , "Clinical Approval Document" and other staged approvals before finally obtaining the new drug certificate.
When talking about the technical advantages of Zhongkebaike, Chen Huangshi, who was originally a little reserved, suddenly became eloquent. He told us that Zhongkebaike has a new drug research and development platform. On this platform, He summed up "three highs and one low"!
First, the success rate of research and development is high - after decades of accumulation of technical experience, we have established a comprehensive system covering gene discovery, gene recombination, large-scale fermentation, protein refolding, protein purification, biological activity detection methods, and preparations. A one-stop research and development platform for advanced biotechnology and rapid conversion to production. According to statistics, the average time from project establishment to the development of laboratory products is 4-6 months, and the project success rate is as high as 80%;
Second, the product has high technical content - during the research and development process, we pay close attention Advanced technologies, especially human drug research and development and product updates, are introducing new technologies and products into the field of veterinary drugs at any time. Thanks to years of technology accumulation in the R&D platform, the process technology of our products has the characteristics of "high density, high expression, and high yield", and has higher output and efficiency than other similar products;
The third is Product efficacy is high - using this platform, we have successfully developed dozens of products, representing the recombinant canine interferon product, which is also the most active canine interferon to date. Compared with other similar products, this product has better clinical efficacy, lower side effects, and an overall cure rate increased by 10-15%, which is also the core competitiveness of the company.
"One low"
is the low production cost of the product - we proposed at the beginning of the research that "the process suitable for industrial production is the qualified process". In the production process, Reduce production costs through continuous technological optimization and innovation. In order to meet the requirements of large-scale industrial production, it also meets the needs of the veterinary drug industry.
When we asked if we would encounter difficulties in research and development, Chen Huangshi smiled and said: "The research and development process will inevitably encounter various difficulties. As a developer, when encountering difficulties, "Solving difficult problems is one of the greatest joys of our work and the greatest source of accomplishment." When encountering difficulties, Chen Huangshi will lead everyone to brainstorm, consider solutions and try multiple process routes. At the same time, he will also seek help from external partner institutions to jointly overcome technical difficulties. Speaking of his most unforgettable experience, Chen Huangshi recalled the Mid-Autumn Festival in 2015. At that time, the company encountered technical problems. Chen Huangshi led the R&D team to stay in the laboratory for more than 2 months until the problem was solved, even during the Mid-Autumn Festival and National Day holidays.
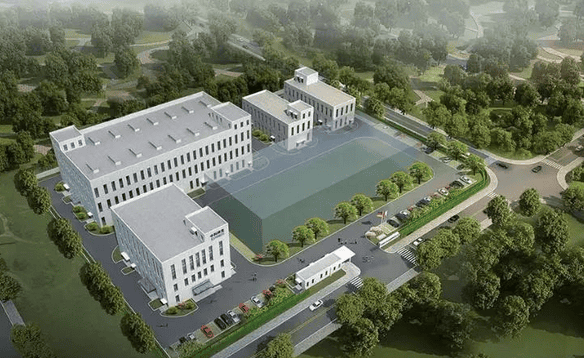
In order to adapt to the company's development needs, in 2018, Zhongkebaike was in contact with the Science and Technology City for only one week before registering and establishing a wholly-owned subsidiary - Zhongkebaike in Tianjin Beijing-Tianjin Zhongguancun Science and Technology City Gram (Tianjin) Biopharmaceutical Co., Ltd. Zhongkebaike plans to invest 120 million yuan to build a modern GMP production base for veterinary biological products. The strategic layout of Beijing-Tianjin-Hebei coordinated development is realized with R&D and incubation in Zhongguancun, Beijing, and industrialization and achievement transformation in Baodi, Beijing, Tianjin and Zhongguancun.
The new factory will build a recombinant interferon freeze-dried powder injection production line, a vaccine production line, a detection kit production line, a solid disinfectant packaging line, as well as R&D quality inspection and other supporting facilities. In the early stages of the design, Zhongkebaik decided to build an intelligent workshop to achieve a high degree of automation. Therefore, the equipment purchased are the most automated equipment at home and abroad, such as fully automatic fermentation tank systems, fully automatic liquid preparation and filling production lines, and the most advanced equipment. The freeze-drying machine with automatic feeding and discharging functions, and the packaging line are also fully automated as much as possible to reduce manpower and improve efficiency.
When talking about why he chose Beijing-Tianjin Zhongguancun Science and Technology City as the starting point for another take-off, Chen Huangshi said that firstly, he was attracted by the development model and industrial planning layout of Beijing-Tianjin Zhongguancun Science and Technology City; secondly, he was attracted by the distance between Baodi District and Beijing-Tianjin Zhongguancun Science and Technology City. Beijing has close geographical advantages and transportation advantages. Coupled with the "Haihe Talent Plan" issued by the Tianjin Municipal Government and several major benefit policies for the Beijing-Tianjin Zhongguancun Science and Technology City such as "talent settlement, children's education, and house purchase", it provides great support and help to the companies settled there. Enterprises can attract more talents, enjoy the coordinated development of Beijing, Tianjin and Hebei, and develop here with peace of mind. "Looking back, no matter from which aspect, settling in the Beijing-Tianjin Zhongguancun Science and Technology City was a very correct decision!" Chen Huangshi told us.
When talking about the help provided by Science and Technology City to Zhongkebaike after its establishment, Chen Huangshi was full of praise, "Considering that it is our first time to build a factory and lack experience in building a factory, Baodi District Government Service Center , the Zhongguancun Administrative Committee and the Science and Technology City gave us great help and support. The comrades of the Science and Technology City even assisted us in handling the relevant requirements for the project planning and taught us step by step so that we could complete it on August 16 this year. After the land was delisted, the construction permit was obtained in just 26 working days. "
At present, Zhongkebaike's new factory is under construction and is expected to be completed and accepted in the second half of 2020. After acceptance, the company will apply for and pass GMP certification from the Ministry of Agriculture and officially go into production in the first half of 2021. GMP certification is a set of mandatory standards applicable to pharmaceutical, food and other industries. It requires enterprises to meet health quality requirements in accordance with relevant national regulations in terms of raw materials, personnel, facilities and equipment, production processes, packaging and transportation, quality control, etc., and form a set of Operational operating standards help companies improve the corporate sanitary environment and promptly identify problems in the production process and improve them. In order to meet this standard, the construction cost of the new factory is about 50% higher than that of other ordinary production enterprises. Despite this, it will provide effective guarantee for the quality control of later-produced drugs.

 扫一扫微信交流
扫一扫微信交流
发布评论